- Seismics
- Coring Equipment
- Sediment physics
- Sediment chemistry
- Process rates
- Microbiology
- Molecular biology
- Water column
Sulfide concentration
Methane oxidation
CHEMISTRY
Sulfide concentration
Dissolved sulfide in the water column comprises the forms H2S and HS-. Water is sampled immediately from the CTD-rosette bottles using a plastic tubing and avoiding contact with air.![]()
| A 50 ml water sample is transferred to a 50 ml serum bottle and 4 ml of the appropriately mixed diamine reagent |
Diamine-reagent: dissolve N,N-dimethyl-p-phenylenediamine sulfate and ferric chloride (see table) in 500 ml cool 50 % (v/v) reagent grade hydrochloric acid.
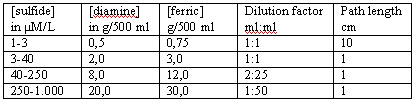 |
| The serum cap is replaced promptly to reduce volatilization of the H2S, and the solution is mixed gently. |
After 20 min the absorbance at 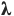 = 670 nm is determined spectrophotometrically in the appropriate cuvette. = 670 nm is determined spectrophotometrically in the appropriate cuvette.
|
| The concentration of sulfide in the sample is calculated from C = F (A-Ab) |
back to top